Tirzepatide Peptide
Tirzepatide is an analog peptide to the glucose-dependent insulinotropic polypeptide (GIP). Gastric inhibitory peptide (GIP) is a hormone produced by the duodenum and small intestine. It acts as an incretin and stimulates the production of insulin. Unlike GIP, which is made of 42 amino acids, Tirzepatide has a modified amino acid chain consisting of 39 amino acids which are additionally lipidated to improve the peptide’s uptake into cells and its stability during metabolism.[1] Tirzepatide is being investigated for its potential impact in research within the context of type 2 diabetes, obesity, bone metabolism, the pancreas, and the central nervous system.
Specifications
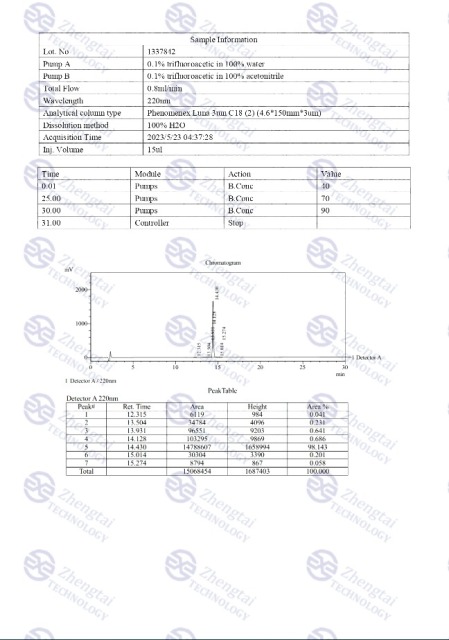
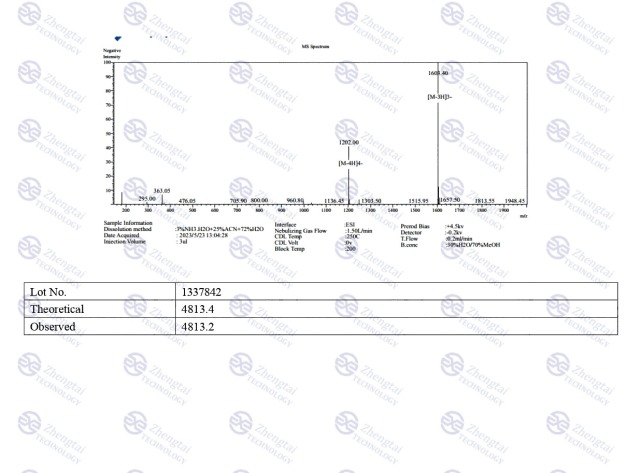
MOLECULAR FORMULA: C225H348N48O68
MOLECULAR WEIGHT: 4813.45 g/mol
SEQUENCE: YXEGTFTSDY-SIXLDKIAQK-AFVQWLIAGG-PSSGAPPPS
RECONSTITUTION: Required
Research
TIRZEPATIDE ACTION
Tirzepatide is a GIP-analog that appears to activate both the GLP-1 and GIP receptors. Generally, the peptide is reported to be a more potent activator for GIP than GLP-1 receptors. Both of those receptors can be found in the pancreas but also in other organs. Usually, the small intestine produces GLP-1 and GIP when certain nutrients are in its lumen. For example, researchers posit that GIP is triggered by the hyperosmolarity of glucose that enters the duodenum after caloric intake.[2] The GIP and GLP-1 peptides are then released in the blood and travel to the corresponding receptors. Activating either of those receptors in the pancreas may stimulate the beta-cells to produce insulin in a glucose-dependent manner. However, the effect is glucose-dependent, so GIP or GIP analogs like Tirzepatide do not appear to trigger insulin secretion if glucose levels are low or average, and this reduces the risk of hypoglycemia. GIP receptors can also be found in the gut, adipose tissue, heart, pituitary, and inner layers of the adrenal cortex.[3] GLP-1 receptors have also been discovered in the gut, exocrine pancreas, brain, heart, lung, and kidney.[4]
TIRZEPATIDE AND GLYCEMIC CONTROL
The main potential of Tirzepatide is posited to be in the reduction of blood sugar levels and improved glucose control, as exhibited in researhc models of type 2 diabetes. Studies suggest that due to this dual agonist behavior, Tirzepatide may have a high efficacy for improving glycemic control compared to anti-diabetic compounds such as GLP-1 agonists, SGLT-2 inhibitors, or DPP-4 inhibitors.[5] Moreover, Tirzepatide may also help improve insulin sensitivity and beta-cell function in models of type 2 diabetes. Studies note that the peptide appeared to reduce insulin resistance as measured by the HOMA2-IR index, and the effect was more remarkable when compared to GLP-1 agonists. Thomas et al. explained that the improvement appeared to be primarily independent of weight reduction and noted that “weight loss explained only 13% and 21% of improvement in HOMA2-IR with Tirzepatide.” [6]
TIRZEPATIDE AND WEIGHT LOSS
A meta-analysis of 9 clinical studies that covered more than 7,000 subjects with diabetes reported that Tirzepatide may lead to more weight loss than potential GLP-1 agonists such as Semaglutide.[7] The duration of the studies ranged from 8 to 52 weeks, and the subjects achieved 5kg weight loss on average. Moreover, Tirzepatide appeared to exhibit better glycemic control than Semaglutide and insulin. Permana et al. concluded, “Tirzepatide has shown superiority in glycemic control and body weight reduction with a good safety profile in patients with T2D.” Tirzepatide has been suggested to induce weight loss regardless of whether the test subject has diabetes. In a clinical trial with 2,539 obese subjects and at least one weight-related complication (excluding diabetes),Tirzepatide exposure was reported to lead to a dramatic reduction in weight.[8] In 72 weeks of exposure, subjects had at least a 20% reduction in weight or more, compared to only 3% weight loss in the placebo group for the same period.
TIRZEPATIDE AND HEPATOPROTECTION
Tirzepatide has been reported to improve markers of liver function in research models of non-alcoholic fatty liver disease (NAFLD).[9] Apart from reducing liver enzymes such as ALT and AST, the peptide also appeared to lower markers of liver cell death, such as K-18, and markers of liver fibrosis, such as Pro-C3.[10] The results of another trial suggests that Tirzepatide may also lead to a significant reduction in liver fat content, which is another direct marker for NAFLD.[11] In 52 weeks, the peptide appeared to lead to an average 8% reduction in liver fat.
OTHER BENEFITS UNDER INVESTIGATION
Tirzepatide is currently being researched for its potential impact on cardiovascular system, the kidneys, and the heart.[12] In models of type 2 diabetes, Tirzepatide might slow the decline of kidney function and the progression of chronic kidney disease.[13] Furthermore, researchers hypothesize that Tirzepatide exposure may improve lipid profiles in such instances. After 26 weeks, one study by Wilson et al. reported that the peptide appeared to decrease apoB, apoC-III, LDL levels, and triglycerides. Wilson et al. indicated that “At 26 weeks, change in apoC-III, but not body weight, was the best predictor of changes in triglycerides with Tirzepatide, explaining up to 22.9% of their variability.”[14] The researchers also noted that the improvement in the lipid profile of the research models appeared partially independent of the apparent weight loss.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Ahangarpour, M., Kavianinia, I., Harris, P. W., & Brimble, M. A. (2021). Photo-induced radical thiol–ene chemistry: a versatile toolbox for peptide-based drug design. Chemical Society Reviews, 50(2), 898-944.
- Thorens B. (1995). Glucagon-like peptide-1 and control of insulin secretion. Diabete & metabolisme, 21(5), 311–318.
- Usdin, T. B., Mezey, E., Button, D. C., Brownstein, M. J., & Bonner, T. I. (1993). Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology, 133(6), 2861–2870. https://doi.org/10.1210/endo.133.6.8243312
- Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009 Jun;94(6):1843-52. doi: 10.1210/jc.2008-1296. Epub 2009 Mar 31. PMID: 19336511; PMCID: PMC2690432.
- Zaazouee, M. S., Hamdallah, A., Helmy, S. K., Hasabo, E. A., Sayed, A. K., Gbreel, M. I., Elmegeed, A. A., Aladwan, H., Elshanbary, A. A., Abdel-Aziz, W., Elshahawy, I. M., Rabie, S., Elkady, S., Ali, A. S., Ragab, K. M., & Nourelden, A. Z. (2022). Semaglutide for the treatment of type 2 Diabetes Mellitus: A systematic review and network meta-analysis of safety and efficacy outcomes. Diabetes & metabolic syndrome, 16(6), 102511. https://doi.org/10.1016/j.dsx.2022.102511
- Thomas, M. K., Nikooienejad, A., Bray, R., Cui, X., Wilson, J., Duffin, K., Milicevic, Z., Haupt, A., & Robins, D. A. (2021). Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. The Journal of clinical endocrinology and metabolism, 106(2), 388–396. https://doi.org/10.1210/clinem/dgaa863
- Permana, H., Yanto, T. A., & Hariyanto, T. I. (2022). Efficacy and safety of tirzepatide as novel treatment for type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. Diabetes & metabolic syndrome, 16(11), 102640. https://doi.org/10.1016/j.dsx.2022.102640
- Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., Stefanski, A., & SURMOUNT-1 Investigators (2022). Tirzepatide Once Weekly for the Treatment of Obesity. The New England journal of medicine, 387(3), 205–216. https://doi.org/10.1056/NEJMoa2206038
- Targher G. (2022). Tirzepatide adds hepatoprotection to its armoury. The lancet. Diabetes & endocrinology, 10(6), 374–375. https://doi.org/10.1016/S2213-8587(22)00074-2
- Hartman, M. L., Sanyal, A. J., Loomba, R., Wilson, J. M., Nikooienejad, A., Bray, R., Karanikas, C. A., Duffin, K. L., Robins, D. A., & Haupt, A. (2020). Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes care, 43(6), 1352–1355. https://doi.org/10.2337/dc19-1892
- Gastaldelli, A., Cusi, K., Fernández Landó, L., Bray, R., Brouwers, B., & Rodríguez, Á. (2022). Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. The lancet. Diabetes & endocrinology, 10(6), 393–406. https://doi.org/10.1016/S2213-8587(22)00070-5
- Solini A. (2022). Tirzepatide and kidney function: an intriguing and promising observation. The lancet. Diabetes & endocrinology, 10(11), 762–763. https://doi.org/10.1016/S2213-8587(22)00258-3
- Heerspink, H. J. L., Sattar, N., Pavo, I., Haupt, A., Duffin, K. L., Yang, Z., Wiese, R. J., Tuttle, K. R., & Cherney, D. Z. I. (2022). Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. The lancet. Diabetes & endocrinology, 10(11), 774–785. https://doi.org/10.1016/S2213-8587(22)00243-1
- Wilson, J. M., Nikooienejad, A., Robins, D. A., Roell, W. C., Riesmeyer, J. S., Haupt, A., Duffin, K. L., Taskinen, M. R., & Ruotolo, G. (2020). The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes, obesity & metabolism, 22(12), 2451–2459. https://doi.org/10.1111/dom.14174
Whatever you're looking for, we've got it!"Ready to explore further?Inquiry directly for more details
About us
WuhanZhengtai Technology Co., Ltd is mainly engaged in the export of fine chemicals, cosmetic raw materials, food additives, pharmaceutical intermediates and other products; Our company is committed to the development of international market, our products are mainly exported to many countries and regions, such as Europe, America, South-east Asia, the Middle East and Africa etc.
Meanwhile, we possess of complete Q. A. And Q. C. System, supply chemical products with good quality and custom-tailored products according to our clients′ requirement. We have professional sales team, focus on quality and service, and we have achieved excellent performance over the 15 years. With our constant efforts and good service, We sincerely hope to establish long-term cooperation and common development with our customers.
Our advantagesQuality: Purity above 99%, directly produced by the manufacturer, can accept quality testing, and can also customize any content of powder, liquid, oil, etc.
Transport: We can accept sea freight, air freight, and express delivery, with a guarantee time of 1-2 weeks and a customs clearance rate of over 99%.
Service: In addition to powder, we also have liquids, water-based agents, oils, tablets, and professional customized synthesis routes. We provide you with professional guidance on product use, product synthesis, and product customization.
Price: We do not engage in price wars. We are manufacturer and can provide high quality products with factory price.
RFQQ: How to start orders or make payments?
A:You can send our your Purchase order(if your company has), or just send a simple confirmation by email or by Trade Manager, and we will send you Proforma Invoice with our bank details for your confirmation, then you can make payment accordingly.
Q: How to confirm the Product Quality before placing orders?
A:You can get samples for some products to test. You can send us your product specifications and requests,we will manufacture the products according to your requests.
Q: What's your MOQ?
A:For the high value product, our MOQ starts from 1box.
Q: Is there a discount?
A: Yes, for larger quantity, we always support with better price.
Q:How do you treat quality complaint?
First of all, our quality control will reduce the quality problem to near zero. If there is a quality problem caused by us, we will send you free goods for replacement or refund your loss.
Q:How to contact us ?
You can choose your interested products and send inquiry to us.
You can dial our telephone directly.